We are an owner-managed family company based in Germany. In addition to high quality standards, we rely on basic research and scientific innovations. Our high professional specialization and decades of experience make us a holistic competence partner for cleaning and sterilization monitoring. At the same time treating people and nature with respect is very important to us.

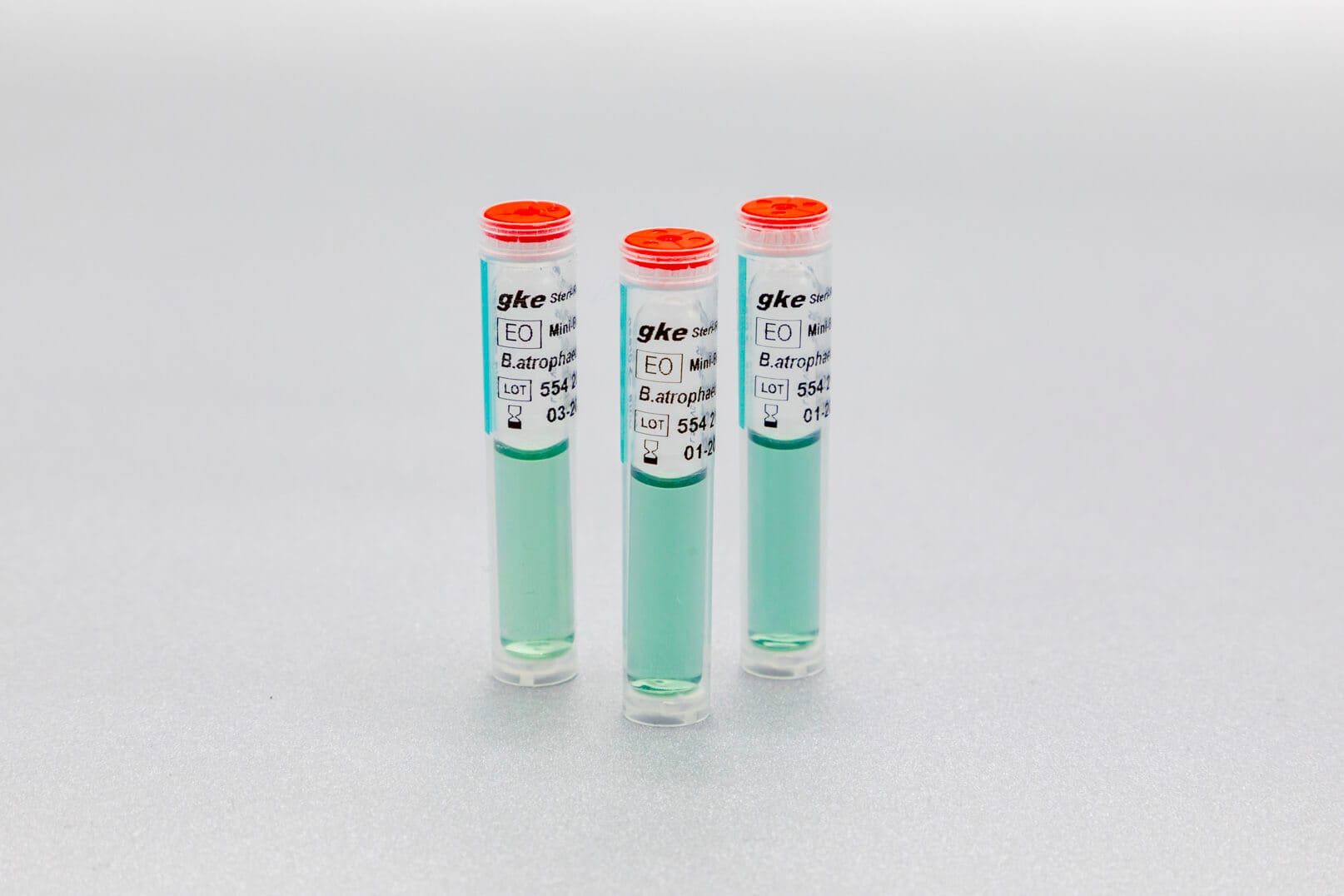
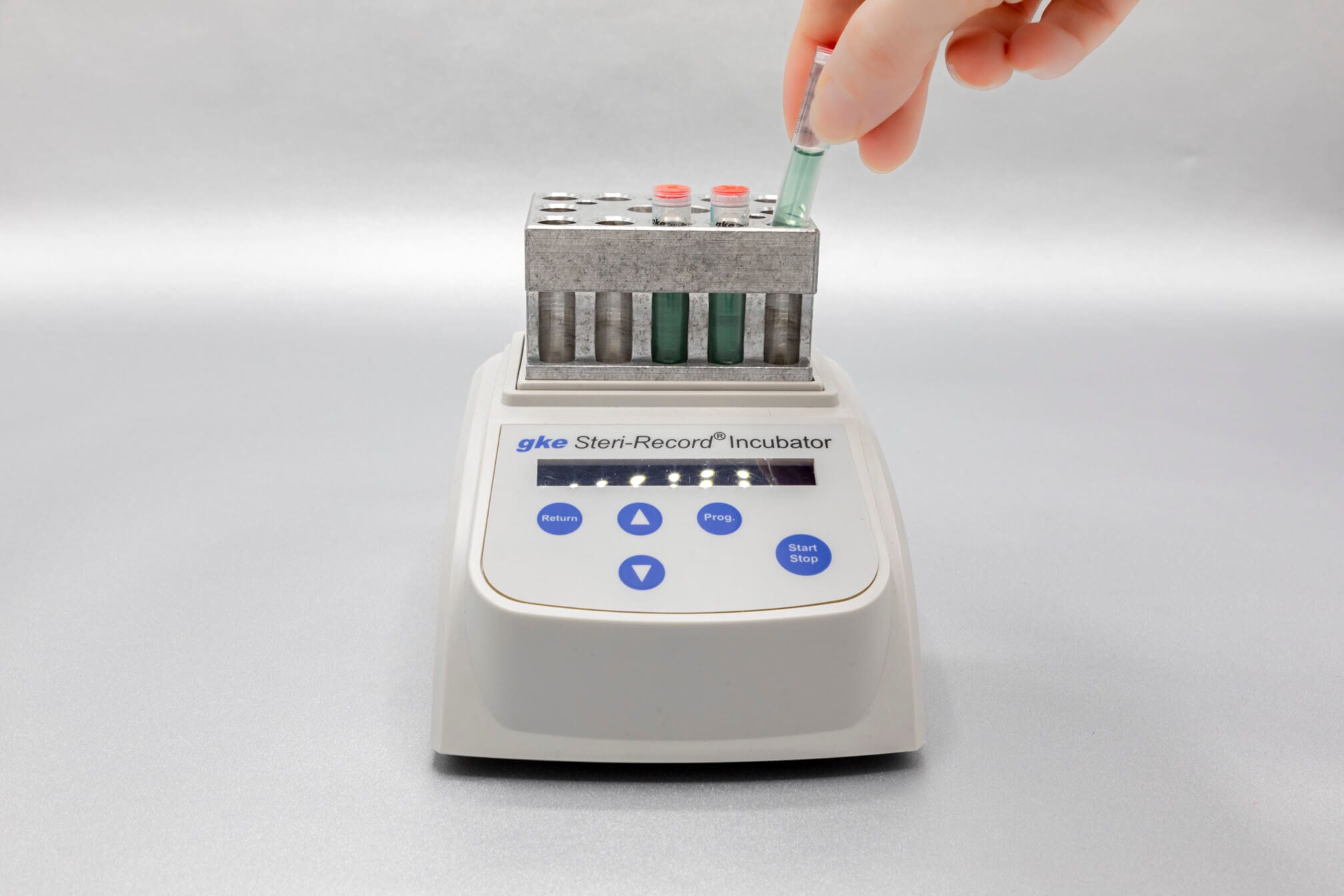
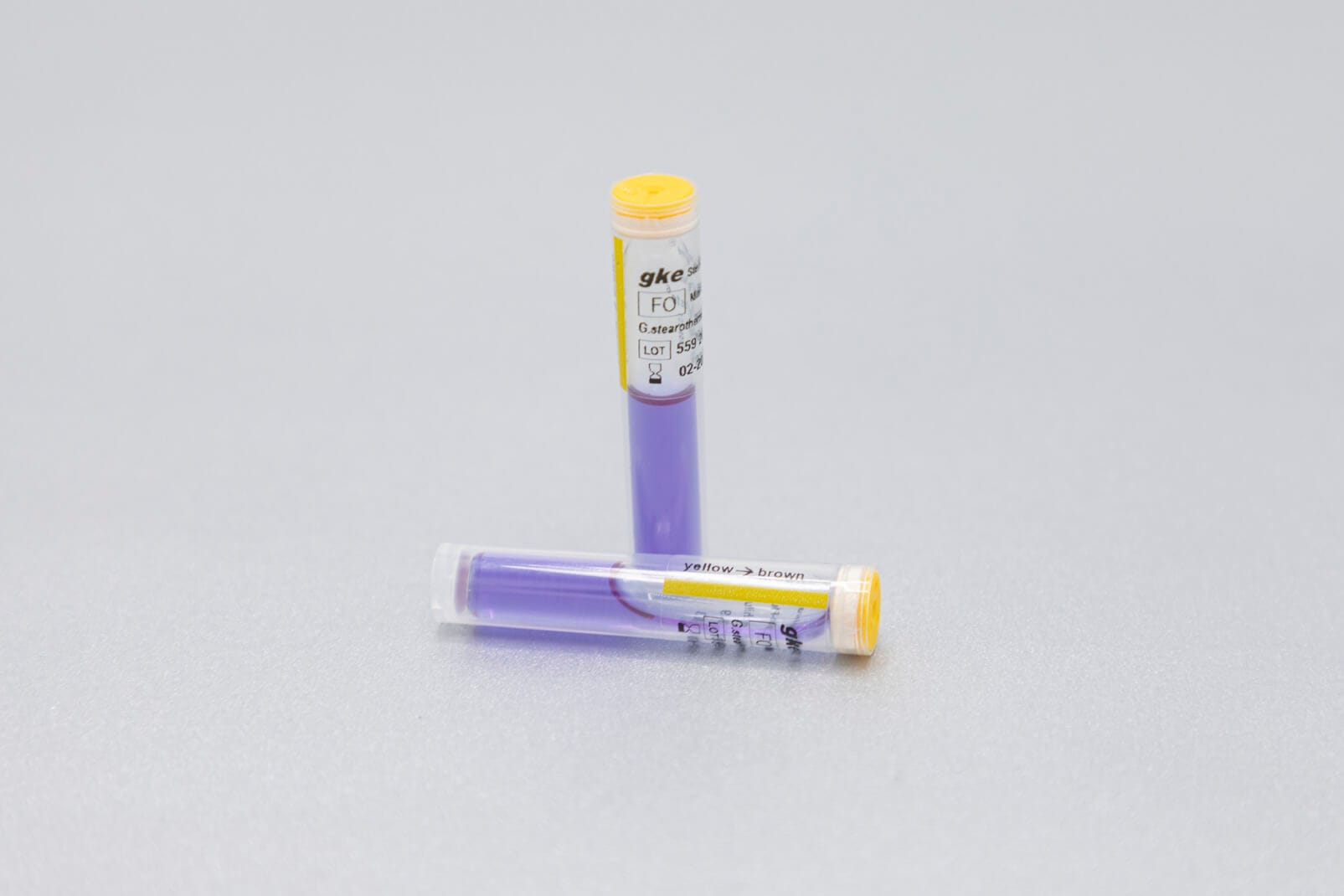
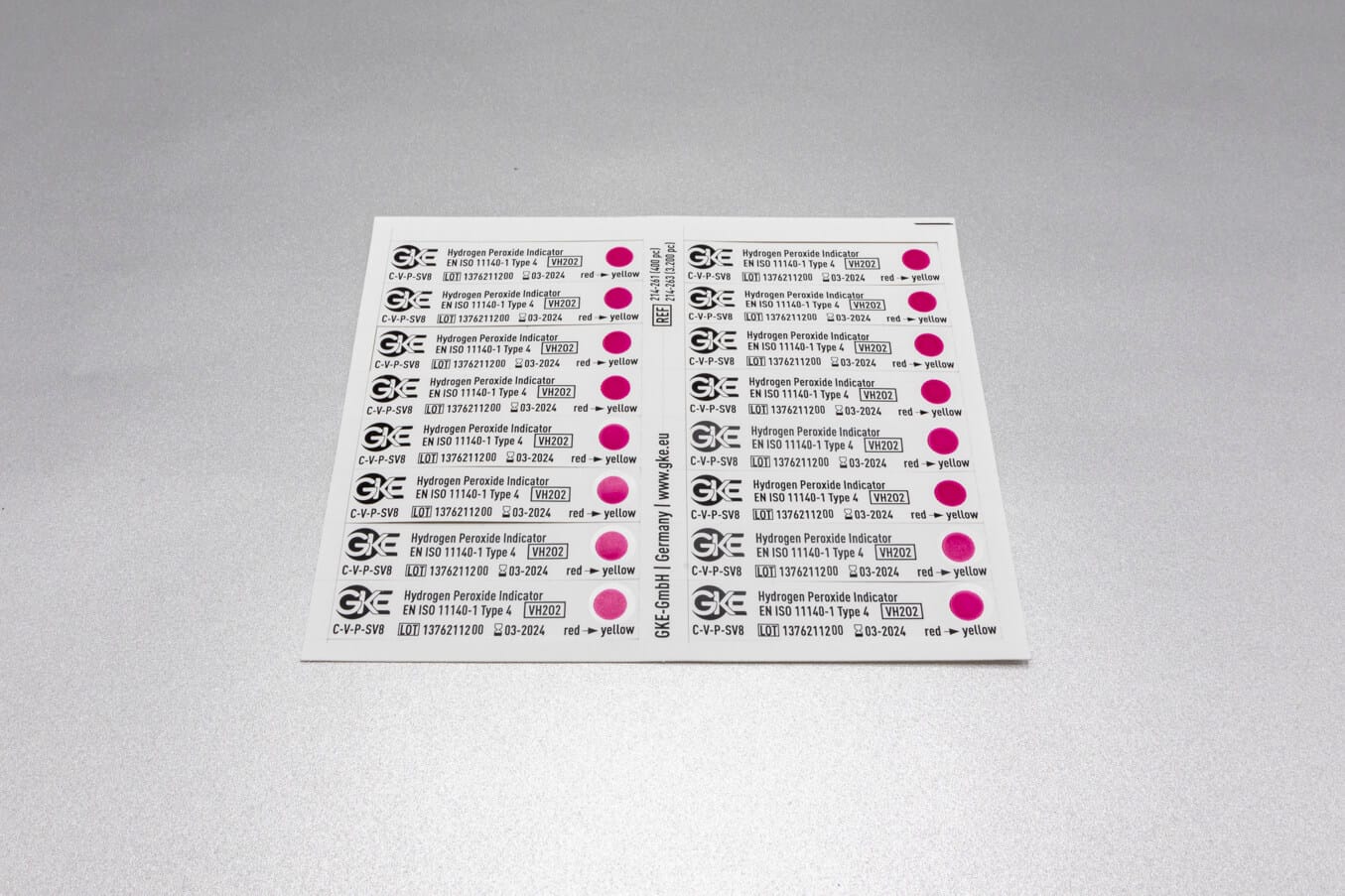
We develop concepts for the cleaning and sterilization process monitoring for the preparation of medical devices and produce the corresponding biological and chemical indicators. We are happy to help you with the design of the monitoring with biological and chemical indicators and the selection of suitable test objects process challenge devices (PCDs).
We offer you hands-on science.

Mesa Laboratories, Inc. a global leader in the design and manufacturing of life science tools and critical quality control solutions, today announced the completed acquisition of GKE-GmbH’s sterilization indicators business and its accredited, independent testing lab SAL GmbH. Additionally, Mesa has entered into a definitive agreement to purchase GKE’s Chinese sales entity, Beijing GKE Science & Technology Co. LTD (“GKE China”).
For more information about the Company, please visit its website at www.mesalabs.com
Read more …
The GKE website is now available in three languages - English, German and now Chinese, and can be changed by clicking on the language shortcuts.
The website provides easy access to corporate, product and technical information that is also scalable for mobile devices.
Read more …
When reprocessing medical devices (cleaning, disinfection, sterilization), the reprocessing process must be optimally designed for the specific instruments and monitored on a daily basis.
A new information brochure is now available, which presents all information and products relevant to reprocessing in medical and dental practices in a clear format.
Read more …
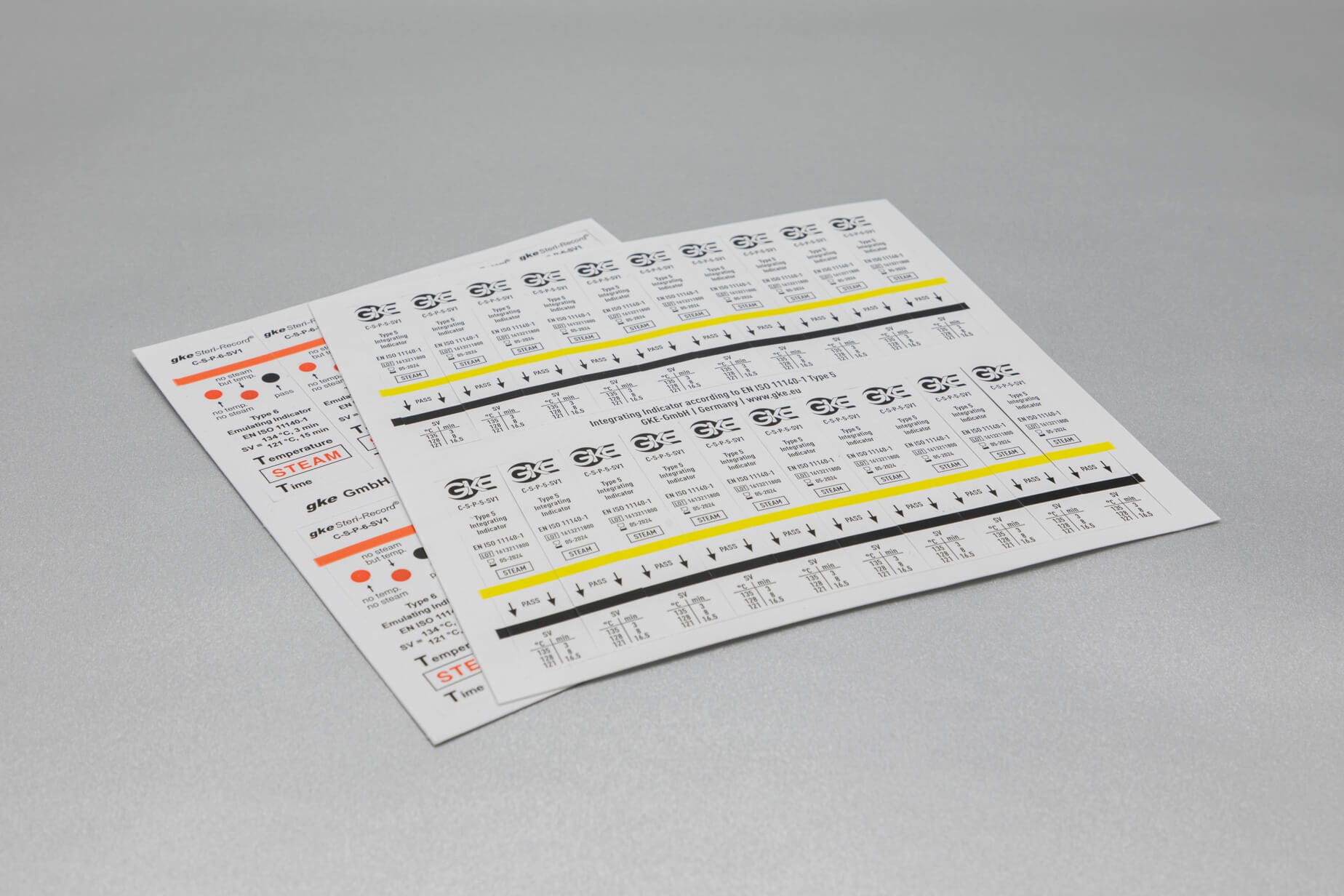
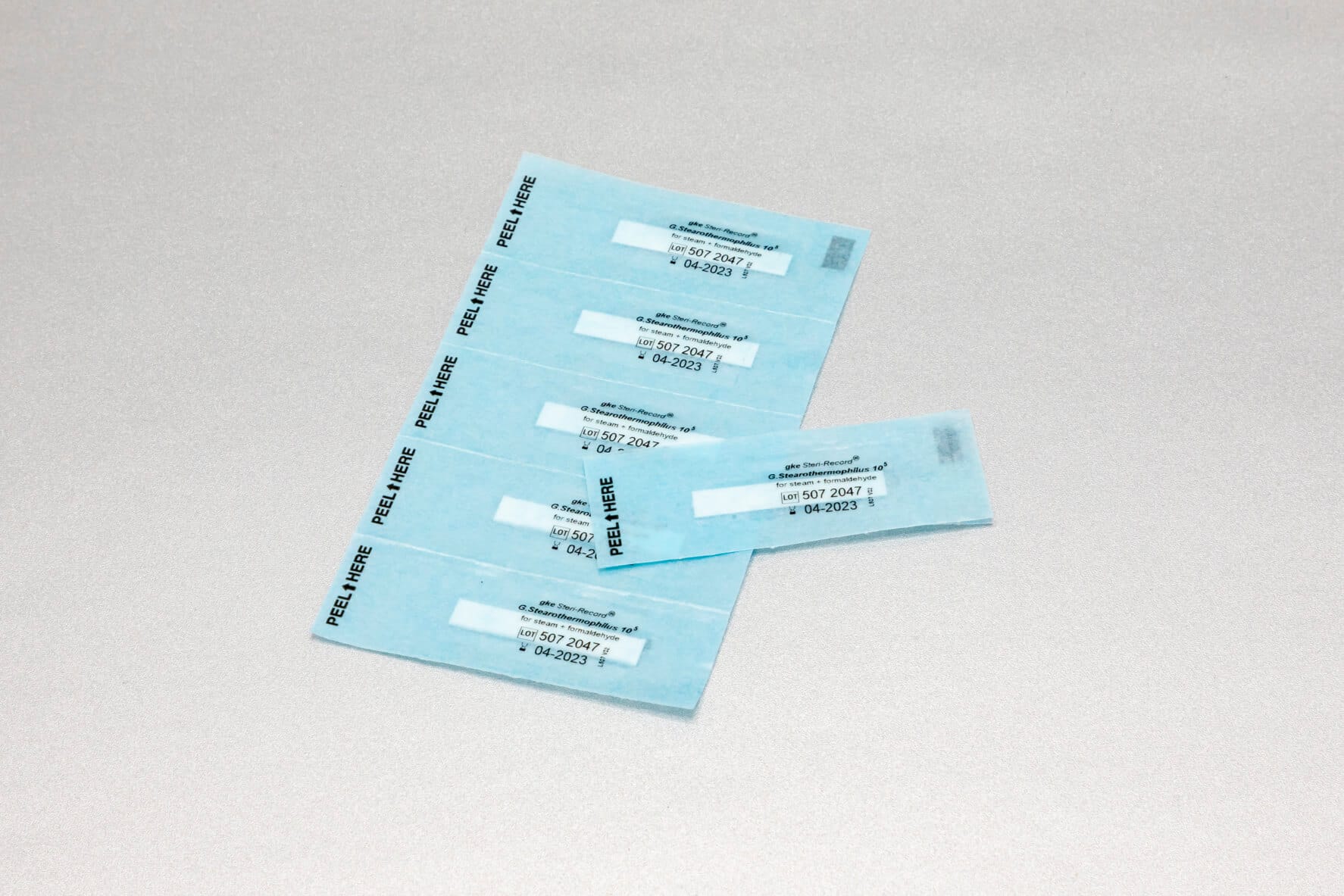
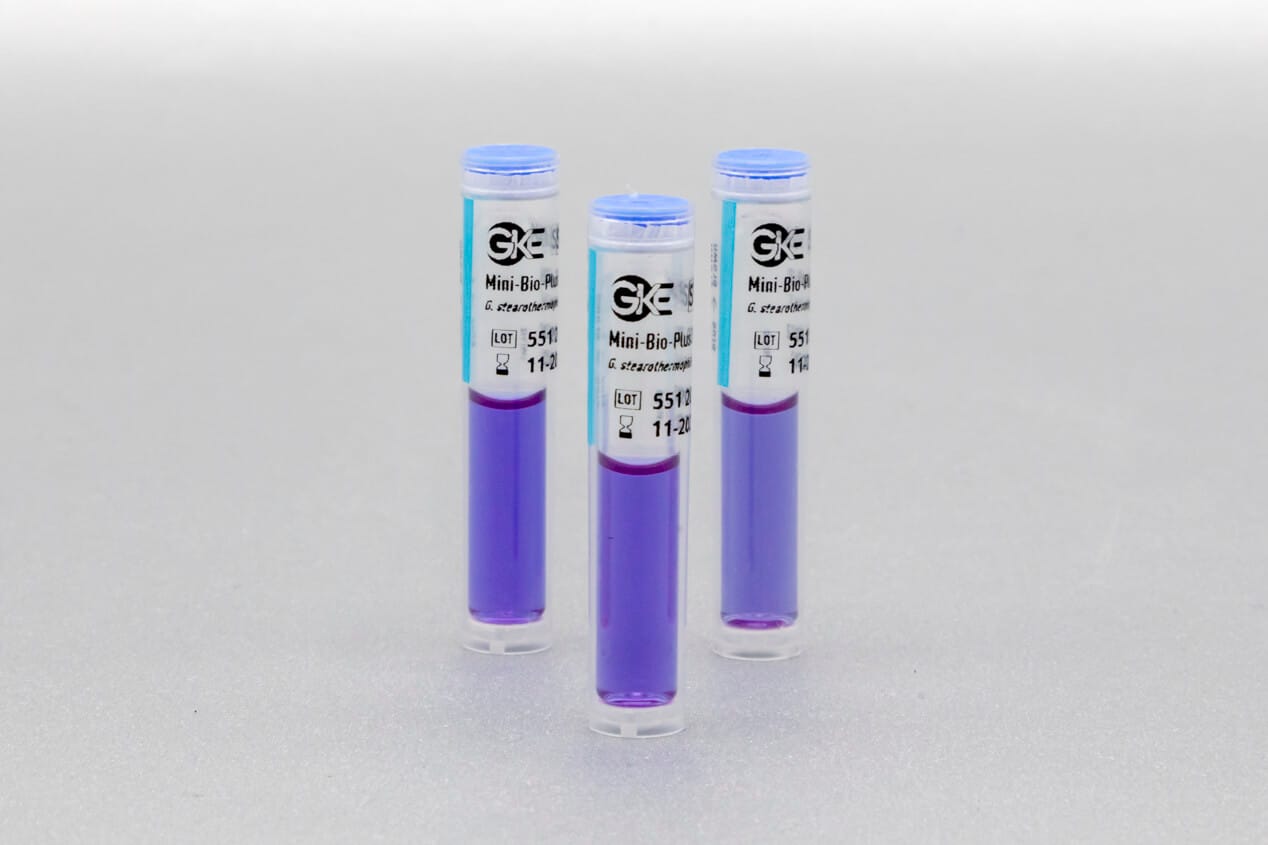
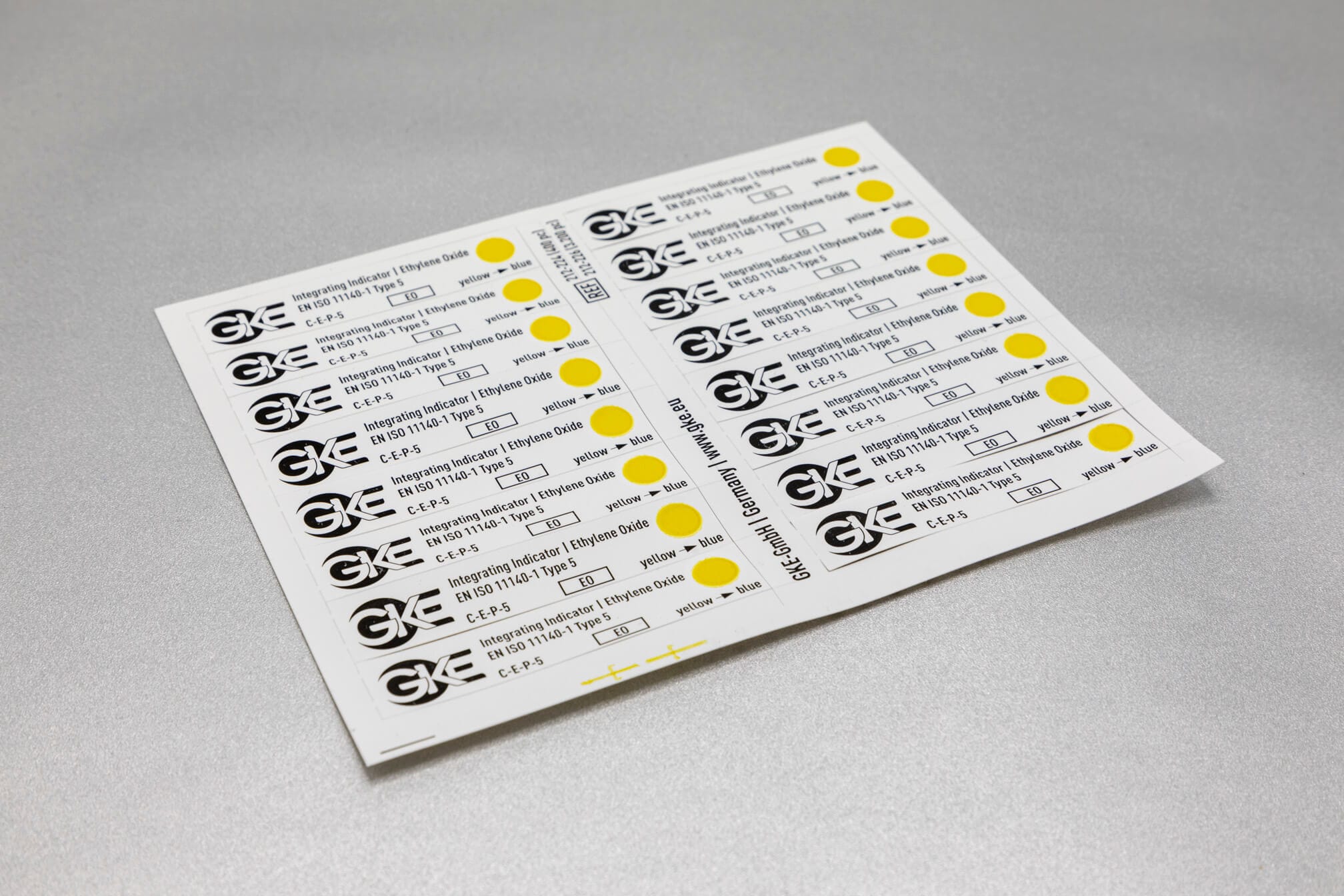
Adapted to the respective sterilization process, we offer special, chemical and biological indicators as well as process challenge devices (PCDs). There are different systems for routine monitoring, documentation or validation.
If you have any questions regarding the right product selection for your needs and your requirement profile, we are happy to help you. Get in touch with us using our contact form or give us a call.